volume_mute
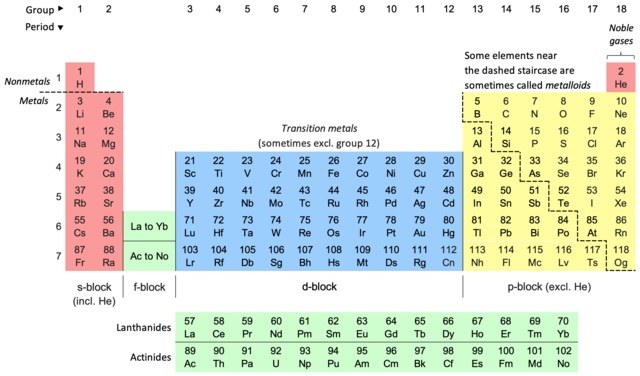
With elements of which column would you expect column 2 elements to unite in a 1-to1 ratio?
publish date: 2024/06/06 21:46:16.863128 UTC
volume_mute
Correct Answer
16
Explanation
Column 2 elements have two extra electrons in their outer shells. The elements of column 16 lack two electrons from having full shells.
The discussion of the number of electrons allowed in various orbits and the methods by which atoms combine to form molecules is not always as simple as indicated here. These simple methods work only for atoms of small atomic number.
Reference
Basic Physics: A Self-Teaching Guide
